K3 Fe(Cn)6 Oxidation Number / Oxidation Number Of K3 Fe Cn 6 : The oxidation number is synonymous with the oxidation state.
K3 Fe(Cn)6 Oxidation Number / Oxidation Number Of K3 Fe Cn 6 : The oxidation number is synonymous with the oxidation state.. This cyano group is virtually identical to the cyano group which is bound to hydrogen in hcn. An anionic complex is the hexacyanide of the ferric iron (fe +3) ion, the hexacyanoferrate(3−) ion, fe(cn) 6 3−, or. In the complex k 3 f e(c n)6 k (potassium) is alkaline metal with +1 oxidation state,then for the k 3 it's +3. {eq}\displaystyle \rm k_3fe(cn)_6 {/eq} oxidation state: ∴ 3 ( + 1 ) + x + 6 ( − 1 ) = 0
Crystalline salts containing complex ions include potassium hexacyanoferrate(3−) (potassium ferricyanide), k 3 fe(cn) 6, and the hexahydrate of nickel chloride, hexaaquanickel(2+) chloride, ni(h 2 o) 6cl 2. The most common salt of this anion is potassium ferricyanide, a red crystalline material that is used as an oxidant in organic chemistry. Be sure to remember the sign. (i) k 3 co(c 2 o 4) 3 the central metal ion is co. Access detailed answers to various other science and maths questions at byju's.
Therefore, the oxidation number of chromium must be the same as the charge of the complex ion, +3.
The nitrogen is part of the cyano group which is bound to fe. Let oxidation number of f e be x. Be sure to remember the sign. The d orbital occupation for co 3+ is t 2g 6 e g 0. The oxidation number is synonymous with the oxidation state. Causes serious eye irritation warning serious eye damage/eye irritationh335 (17.18%): Chloride ions are behaving as ligands. Determining oxidation numbers from the lewis structure (figure 1a) is even easier than deducing it from the molecular formula (figure 1b). The oxidation number of cu in the complex ion is +2. The most common salt of this anion is potassium ferricyanide, a red crystalline material that is used as an oxidant in organic chemistry. The cv experiment can provide important information about the oxidation state of an element in a The oxidation number of each atom can be calculated by subtracting the sum of lone pairs and electrons it gains from bonds from the number of. Assuming the oxidation state of 'fe' to be 'x' total charge present on the complex is 0.
Many inorganic compounds contain elements that may take on several different oxidation states. The oxidation state can be. Access detailed answers to various other science and maths questions at byju's. Determining oxidation numbers from the lewis structure (figure 1a) is even easier than deducing it from the molecular formula (figure 1b). It is also called hexacyanoferrate (iii) and in rare, but systematic nomenclature, hexacyanidoferrate (iii).

The nitrogen is part of the cyano group which is bound to fe.
Determining oxidation numbers from the lewis structure (figure 1a) is even easier than deducing it from the molecular formula (figure 1b). The cv experiment can provide important information about the oxidation state of an element in a In the complex k 3 f e(c n)6 k (potassium) is alkaline metal with +1 oxidation state,then for the k 3 it's +3. Crystalline salts containing complex ions include potassium hexacyanoferrate(3−) (potassium ferricyanide), k 3 fe(cn) 6, and the hexahydrate of nickel chloride, hexaaquanickel(2+) chloride, ni(h 2 o) 6cl 2. (i) k 3 co(c 2 o 4) 3 the central metal ion is co. K has always +1 charge, since c has 4 valency and n has 3 valency here n is more electronegative hence c 4 electron make bond with 3 electron of n and one electron left for bonding, overall cyanide o.s. The most common salt of this anion is potassium ferricyanide, a red crystalline material that is used as an oxidant in organic chemistry. Causes skin irritation warning skin corrosion/irritationh319 (43.56%): (iv) mn(h 2 o) 6so 4. The compound k3fe(cn)6 is used in calico printing and wool dyeing. In hcn, h has a +1 oxidation number. It is also called hexacyanoferrate (iii) and in rare, but systematic nomenclature, hexacyanidoferrate (iii). Chloride ions are behaving as ligands.
Many inorganic compounds contain elements that may take on several different oxidation states. The iupac name of the coordination compound k 3 fe(cn) 6 is (a) potassium hexacyanoferrate (ii) (b) potassium hexacyanoferrate (iii). Chloride ions are behaving as ligands. The most common salt of this anion is potassium ferricyanide, a red crystalline material that is used as an oxidant in organic chemistry. Its coordination number is 6.
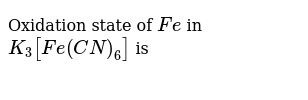
(i) k 3 co(c 2 o 4) 3 the central metal ion is co.
Let oxidation number of f e be x. This cyano group is virtually identical to the cyano group which is bound to hydrogen in hcn. Substituting the value in the following equation: Ferricyanide is the anion fe (cn) 6 3−. Crystalline salts containing complex ions include potassium hexacyanoferrate(3−) (potassium ferricyanide), k 3 fe(cn) 6, and the hexahydrate of nickel chloride, hexaaquanickel(2+) chloride, ni(h 2 o) 6cl 2. Its coordination number is 6. X − 6 = −3. The oxidation number is synonymous with the oxidation state. Therefore, the oxidation number of chromium must be the same as the charge of the complex ion, +3. (i) k 3 co(c 2 o 4) 3 the central metal ion is co. We can obtain this value by removing all the ligands (cn ligands) with their charge. Be sure to remember the sign. Many inorganic compounds contain elements that may take on several different oxidation states.
Komentar
Posting Komentar